Jan, 2015
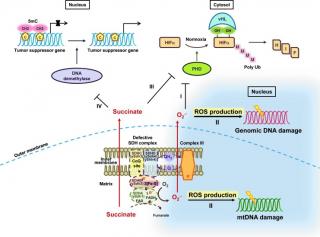
AIMS: Mitochondrial succinate dehydrogenase (SDH) is an essential complex of the electron transport chain and tricarboxylic acid cycle. Mutations in the human SDH subunit D frequently lead to paraganglioma (PGL), but the mechanistic consequences of the majority of SDHD polymorphisms have yet to be unraveled. In addition to the originally discovered yeast SDHD subunit Sdh4, a conserved homolog, Shh4, has recently been identified in budding yeast. To assess the pathogenic significance of SDHD mutations in PGL patients, we performed functional studies in yeast.
RESULTS: SDHD protein expression was reduced in SDHD-related carotid body tumor tissues. A BLAST search of SDHD to the yeast protein database revealed a novel protein, Shh4, that may have a function similar to human SDHD and yeast Sdh4. The missense SDHD mutations identified in PGL patients were created in Sdh4 and Shh4, and, surprisingly, a severe respiratory incompetence and reduced expression of the mutant protein was observed in the sdh4Δ strain expressing shh4. Although shh4Δ cells showed no respiratory-deficient phenotypes, deletion of SHH4 in sdh4Δ cells further abolished mitochondrial function. Remarkably, sdh4Δ shh4Δ strains exhibited increased reactive oxygen species (ROS) production, nuclear DNA instability, mtDNA mutability, and decreased chronological lifespan.
INNOVATION AND CONCLUSION: SDHD mutations are associated with protein and nuclear and mitochondrial genomic instability and increase ROS production in our yeast model. These findings reinforce our understanding of the mechanisms underlying PGL tumorigenesis and point to the yeast Shh4 as a good model to investigate the possible pathogenic relevance of SDHD in PGL polymorphisms.