Jun, 2022
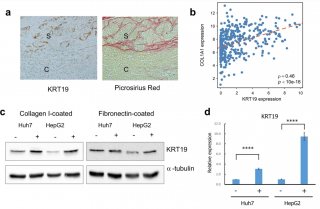
Intrahepatic cholangiocarcinoma (ICC) is characterized by fibrous stroma and clinical behavior more aggressive than that of hepatocellular carcinoma (HCC). Scirrhous HCC is a subtype of HCC with fibrous stroma, frequently has partial cholangiocytic differentiation, and is more likely to have an aggressive behavior. This study explored the interaction of liver cancer cells with the extracellular matrix. Liver cancer cells grown on collagen 1-coated plates showed upregulation of cholangiocytic marker expression but downregulation of hepatocytic marker expression. Three-dimensional sphere culture and Boyden chamber assay showed enhanced invasion and migration ability in collagen 1-conditioned liver cancer cells. Interaction with collagen 1 reduced liver cancer cell proliferation. RNA sequencing showed that in the liver cancer cells, collagen 1 upregulated cell cycle inhibitor expression and cell-matrix interaction, tumor migration, and angiogenesis pathways, but downregulated liver metabolic function pathways. Cholangiocytic differentiation and invasiveness induced by collagen 1 was mediated by the mitogen-activated protein kinase (MAPK) pathway, which was regulated by cell-matrix interaction-induced Src activation. Analysis of the Cancer Genome Atlas cohort showed that collagen 1 induced and suppressed genes were highly enriched in ICC and HCC, respectively. In HCC samples, collagen 1-regulated genes were strongly coexpressed and correlated with COL1A1 expression. Liver cancer cell-matrix interaction induces cholangiocytic differentiation and switches liver cancer cells from a proliferative to an invasive phenotype through the Src/MAPK pathway, which may partly explain the differences in the behaviors of HCC and ICC.